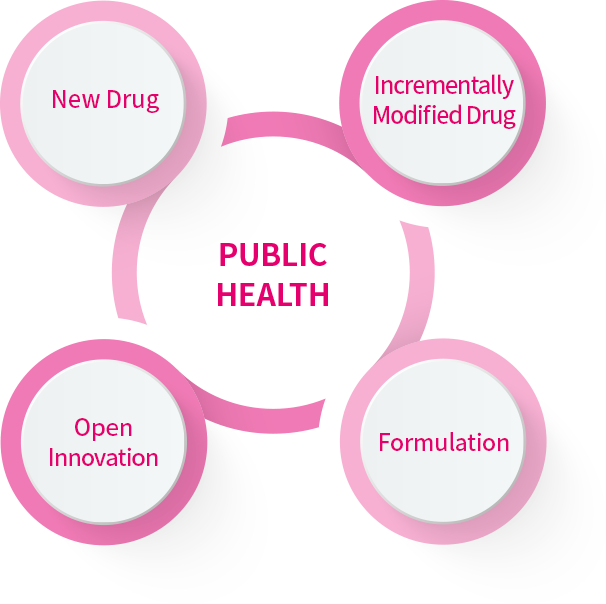
Mother's Pharmaceutical’s R&D Center houses two vital units : the New Drug Development Research Institute and the Product Development Research Institute. The former is centered on the innovation of novel drug formulations, while the latter concentrates on enhancing existing medications and developing generics. These two institutes seamlessly collaborate, fostering a synergistic environment that ensures the highest standards in pharmaceutical development.